Cleaning Diamond Plates
Recent › Forums › Main Forum › Sharpener and Accessory Maintenance › Cleaning Diamond Plates
Tagged: diamond stones, diamonds plates, knife sharpener, sharpener maintenance, sharpening, wicked edge
- This topic has 62 replies, 29 voices, and was last updated 01/21/2019 at 9:21 am by
Steven.
-
AuthorPosts
-
04/26/2016 at 11:00 am #33734
I’ve been working on cleaning some diamond plates up so I could get better images under the microscope. The quest came about in answer to a question in another thread about whether the diamonds themselves can become worn down. So, I started experimenting with some different chemicals that I had around the office. I tried:
- White Vinegar
- Simple Green
- Barkeeper’s Friend
- Rubbing Alcohol
- Ferric Acid (PCB Etchant)
I used the ferric acid full strength, just to try it out. From what I’ve read, when it’s diluted, it shouldn’t harm the nickel plating while still dissolving the steel. The video below shows what happens when you use it full strength. The video is taken under the microscope at 632x and is sped up to 300%:
*Don’t watch this video unless: A) You also like watching paint dry B) Just love sharpening related stuff C) Enjoy science of all sorts.
-Clay
4 users thanked author for this post.
04/26/2016 at 1:50 pm #33739Haha… pretty cool… of course, watching paint dry is my other favorite hobby…
So, did any of the others you tried stand out?
04/26/2016 at 2:24 pm #33740Haha… pretty cool… of course, watching paint dry is my other favorite hobby…
 So, did any of the others you tried stand out?
So, did any of the others you tried stand out? Nothing has really stood out yet but I don’t think I did enough testing yet. In theory, the vinegar should work really well to dissolve the iron, which it would turn to iron acetate. I’ll keep testing stuff and eventually put together a report. Everything did fine as far as making the plates look good to the eye and feel rougher again. It’s more at the microscope level between the stones that I’m working on.
-Clay
04/26/2016 at 4:20 pm #33744I wonder if copper solvent used for firearms would do anything.
04/26/2016 at 4:44 pm #33745I wonder if copper solvent used for firearms would do anything.
It’s definitely on the list to study.
-Clay
04/27/2016 at 1:11 am #33752Outstanding Clay , great idea!, As you all know, I not only love the science of it all but also the not knowing the outcome part. It’s like opening a present as you see the results revealed.
Eddie Kinlen
M1rror Edge Sharpening Service, LLC
+1(682)777-162204/27/2016 at 2:07 pm #33761I spent a little more time on this today, just testing white vinegar with excellent results. I let a dropperful of vinegar sit on a worn 100# plate for 15 minutes and could easily see the solution bubbling away as the acetic acid dissolved the steel and created iron acetate and hydrogen. I then scrubbed the surface of the platen for a few minutes with a wire brush, first with vinegar, then with soapy water until the suds were gone and then gave it a good rinse. Nearly all the metal filings between the stones were gone. One thing I found though is that when the plates are loaded, it’s not just the spaces in between the stones that are covered in metal but the stones themselves. Once I cleaned them well, the stones were much clearer and I could see little pockets where some metal was still left, like looking at a tooth with a filling:
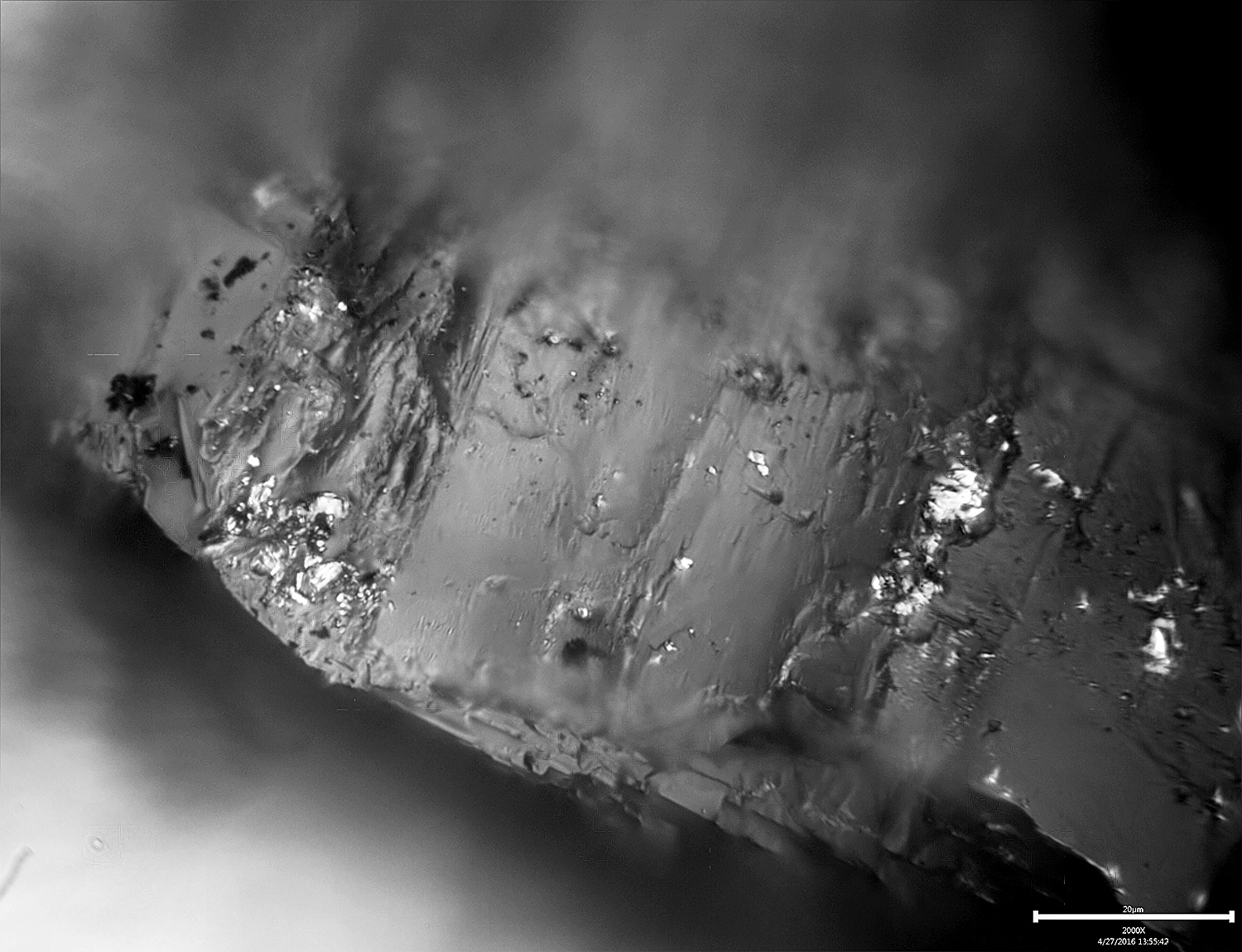
The above image is of a single 100# diamond, taken at 800x as a series of images and then stacked for extended depth of field. The shiny spots are bits of metal embedded in the nooks and crannies of the stone.-Clay
Attachments:
You must be logged in to access attached files.
3 users thanked author for this post.
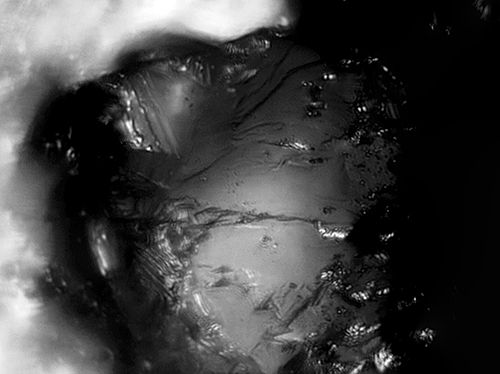
04/27/2016 at 2:15 pm #33763Here’s another stone at 2000x showing bits of metal flakes:
The image is a composite of 25 images that were stacked for extended depth of field.
-Clay
Attachments:
You must be logged in to access attached files.
3 users thanked author for this post.
04/27/2016 at 8:35 pm #33770Very neat Clay!
I’m wondering if cleaning them so thoroughly will offer any improvement in stock removal or finishing of an edge, or if it’s so minute it doesn’t matter?
1 user thanked author for this post.
04/27/2016 at 10:11 pm #33774Ive got the perfect test subject to try that out myself, hopefully by next week if the scope gets here(getting excited!).
My original 100 grit paddle is so worn that heavy pressure is needed just to do anything. I thought it was worn out, but now am wondering if its just clogged flush….interesting.Does this mean that white vinegar is Wicked Edge approved?

2 users thanked author for this post.
04/28/2016 at 8:06 am #33789Does this mean that white vinegar is Wicked Edge approved?

Not quite yet… I need to do more testing to see if the concentration of acetic acid in the vinegar is low enough to not damage the nickel. I’ll get a stone immersed today and let it stay overnight and then have a look on Friday.
-Clay
2 users thanked author for this post.
04/28/2016 at 8:28 am #33790Clay:
Do you have an idea as to how the metal filings are attached to the diamonds – why they don’t just wash off? I could understand where filings of a range of sizes might find pockets in which to lodge themselves, but those small flakes which seem to be attached to the surface of the diamonds? Or are they just loose trash?
04/28/2016 at 11:25 am #33799Clay: Do you have an idea as to how the metal filings are attached to the diamonds – why they don’t just wash off? I could understand where filings of a range of sizes might find pockets in which to lodge themselves, but those small flakes which seem to be attached to the surface of the diamonds? Or are they just loose trash?
I think they’re so small that they are just kind of pasted into the little irregularities on the surface of the stone. Most of the bits of metal are under a micron in width with the biggest one measuring 2.5µ. They’re so thin that they probably flow like plastic when they’re pressed into the stones and stick in pretty well.
-Clay
04/29/2016 at 1:08 pm #33831Great pic, Clay! Since a while I use Bartenders Friend to clean my stones. I had to import it from the UK, but it works noteably better than local cleaning powders, since it contains an acid (oxalic acid). It seems you’ve found an much cheaper ingredient that’s locally available everywhere
 .
.Molecule Polishing: my blog about sharpening with the Wicked Edge
1 user thanked author for this post.
05/02/2016 at 10:47 am #33883Oxalic acid is about four orders of magnitude more acidic than the acetic acid in vinegar, so if you’ve had no issues using barkeeper’s friend, then vinegar should also be safe for the platens.
2 users thanked author for this post.
-
AuthorPosts
- You must be logged in to reply to this topic.