Edge carbides
Recent › Forums › Main Forum › Techniques and Sharpening Strategies › Edge carbides
- This topic has 21 replies, 6 voices, and was last updated 04/15/2017 at 8:54 am by
Readheads.
-
AuthorPosts
-
04/14/2017 at 4:23 am #38405
 I am writing this as a mental rant to generate discussion. I am not sure what I want out of this except maybe a collaborated wiki post which is the closest thing we could get to a peer reviewed journal paper. Below is an image I found on bladeforums. It is endorsed by Lagrangian (ie. Anthony Yan). Pretty fascinating although it is a single piece of evidence and I don’t even know what kind of steel it is, etc. A couple of amateur initial observations:
I am writing this as a mental rant to generate discussion. I am not sure what I want out of this except maybe a collaborated wiki post which is the closest thing we could get to a peer reviewed journal paper. Below is an image I found on bladeforums. It is endorsed by Lagrangian (ie. Anthony Yan). Pretty fascinating although it is a single piece of evidence and I don’t even know what kind of steel it is, etc. A couple of amateur initial observations:- The matrix of martensite and retained austenite is clearly visible
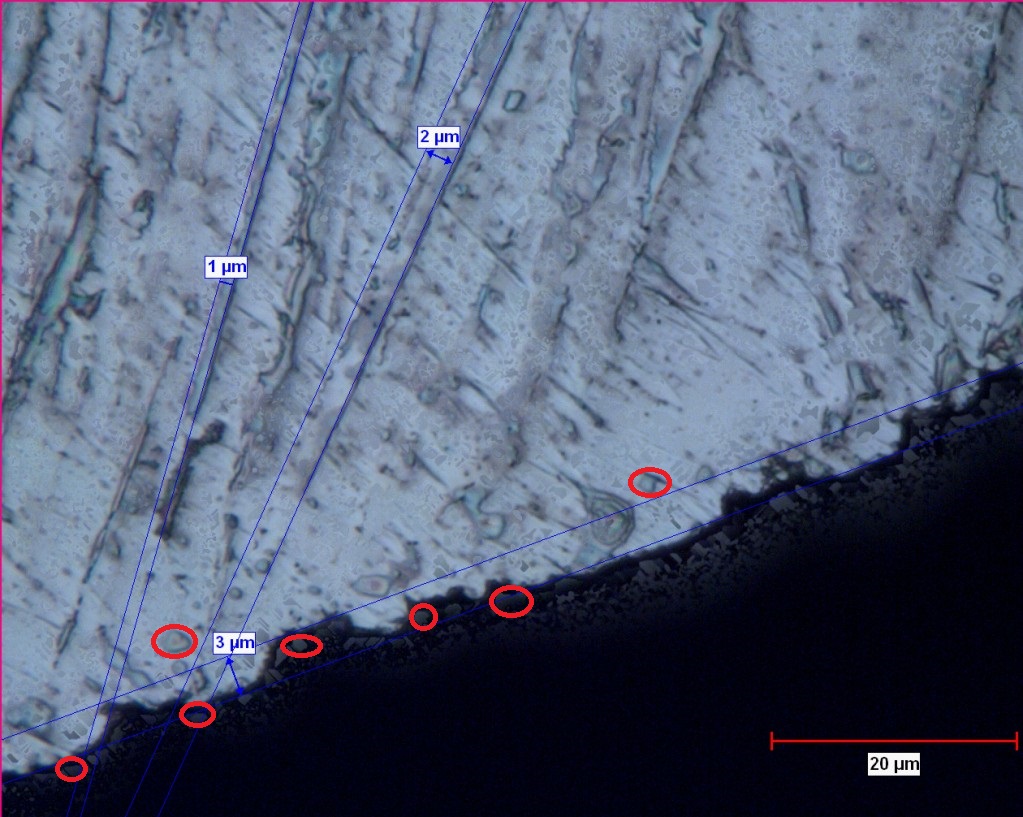
- Primary carbides on the order of 1-5 microns clearly visible
- Secondary carbides in the nanometer range are not visible
- The edge topography “seems” to be a function of the primary carbides.
- The microchips seem to have exposed primary carbides left behind embedded in the martensite
I have little experience with etching to make carbides visible, but the above photograph is very interesting. Readheads, you write that the primary carbides in the order of 1-5 are clearly visible. For me it’s difficult to make out what they are. Can you give a few examples (preferably in this photograph) of these carbides?
Molecule Polishing: my blog about sharpening with the Wicked Edge
04/14/2017 at 6:50 am #38408It may possibly be how the steel is hardened that correlates to the strength of attraction of the carbide in itself, as opposed to it’s attraction to the surrounding matrix. If it’s hardened incorrectly the attraction of the carbide to itself, is greater than the attraction of the carbide to the outside matrix. Then the carbide tends to want to stay whole and the metal can be brittle or chippy as the whole carbide rips out of the matrix. In my experience it may also change as the steel ages. When I was younger I did a lot of spear fishing. We had stainless steel spears made for us by cutting and notching bar stainless steel than had it hardened to give it strength and spring and keep it from bending. When all the spears reached a certain age they all cracked cleanly at the band notch as we used them, one after the other, almost predictably.
Marc
(MarcH's Rack-Its)1 user thanked author for this post.
04/14/2017 at 11:13 am #38418The following is what I think: Carbides are harder than the steel matrix. I figure they must have stronger internal bonds which would make them more likely rip out vs. abrade depending on the volume of steel matrix “holding” them in place. More volume would mean more of the weaker bonds vs. less of the stronger bonds — simple math would dictate which breaks loose first (continued abrasion or micro chip out).
While carbides are preferred for sharpness and wear resistance, I suppose the goal would be to minimize the size of the Primary carbides which are on the grain boundaries. The Secondary carbides are much smaller and my understanding is that they are trapped “within” the grains.
The controlling mechanisms for this are the alloy content AND the specific heat treatments. Verhoeven gets into this fully starting in Ch 8. Its pretty hard reading (as there is no one to ask questions) but studying it makes it clearer each time I do it. The Jay Fisher website is also very good at explaining but he has an obnoxious slant and for some reason seems to ignore Primary carbides. His info seems to relate to Secondary carbides which are in the realm of .001 microns (the good stuff).
If I had the time I would go to Cornell for my Masters/PHD is this. They have new types of electron microscopes which yield real data on electron energy levels and more. I work with the DOD and one of the reasons the space shuttle was not fully replaced by the SSTO (single stage to orbit) is that the materials are not “strong” enough to withstand the heat et al. generated by the thrusts required.
Below is a markup of that picture highlighting the Primary carbides (I think). – LOL
Attachments:
You must be logged in to access attached files.
2 users thanked author for this post.
04/14/2017 at 11:29 am #38421Thanks, Readheads! I wouldn’t have guessed this if you hadn’t pointed the larger carbides out, but now I think I can spot them. And Verhoeven’s work – I would like to Cornell, too, but I have a full-time job and a Ph.D. already, on quite a different subject.
Molecule Polishing: my blog about sharpening with the Wicked Edge
04/15/2017 at 4:07 am #38433Well, armed with only a master degree in music education…
I think Verhoeven is saying from that excerpt that some carbides align better than others in the matrix, and the ones that don’t cause stress. That could be a underlying reason for such drastic changes between “just one more stroke” and complete carbide popout… You essentially have a knife earthquake, the matrix steel is unable to hold the stresses as the edge gets thinner, and it releases and breaks..
As to abrading through the carbides, I believe diamonds and CBN will scratch through the carbides – and popout is also influenced by a cement and concrete problem. Cement is just concrete with no rocks, essentially. Add rocks and you have concrete. If you were to make a triangle shaped concrete and a cement model that represented carbide steel (concrete) and straight carbon steel (cement), you would be able to make a much more pointed apex with the cement since the materials are homogeneous in size. The concrete mixture would require a thicker edge, or more edge geometry to accommodate the size of the rocks. In order to get the dimensions of the two the same, you would need to abrade through the rocks. This exposes both rocks (carbides) and cement until it comes to a point. But the rocks will take up more space along the edge,spaced between now very thin slivers of cement. You could easily knock the edge out, thus simulating carbide popout.
You can also break off the tip of the cement model easily as well. However, I think the thickness of the edge left after the break on the cement would still be small than that of the concrete, which ripped out to the size and thickness of the rocks.
Maybe a little crazy to follow, but you can abrade through carbides to a certain point (no pun intended), but when you approach that critical thickness/thinness, you should either increase the angle or stop abrading in order to avoid carbide popout. Throw in the Verhoeven stress factor (if I read that correctly… that was hard to follow!!), and it further explains what and why carbide popout happens…
04/15/2017 at 7:35 am #38435Maybe a little crazy to follow, but you can abrade through carbides to a certain point (no pun intended), but when you approach that critical thickness/thinness, you should either increase the angle or stop abrading in order to avoid carbide popout. Throw in the Verhoeven stress factor (if I read that correctly… that was hard to follow!!), and it further explains what and why carbide popout happens…
Tom, there is one thing I don’t understand. What do you measn by the critical thickness/thinness, and why should you either increase the angle or stop abrading in order to avoid carbide popout? I’d think that the smaller the abrasive material is, the less chance there is you get a carbide popout. (But maybe I don’t understand Verhoeven well enough.)
Molecule Polishing: my blog about sharpening with the Wicked Edge
04/15/2017 at 8:54 am #38437Therein lies what interests me, as we sharpen thru the progressions we first raise a burr and then continue a scratch removal course of action. The burr raising is done at the coarser grits which is the unavoidable opposite of the apex minimum force goal. While carbides usually don’t pop out during sharpening (except maybe microscopically), we do get microchipping during use, is it at random locations or “weak energy bonding” locations driven by our cutting and chopping in the kitchen.
Hmm, I wonder what the SEM images of steel with no/minimum carbides show. I will look them up for later.
I do not profess that I know what is going on and maybe we are just dealing with the nature of the beast. I will leave for now with the following question: Is it possible to have a steel with ZERO primary carbides and LOTS of secondary carbides.
1 user thanked author for this post.
-
AuthorPosts
- You must be logged in to reply to this topic.